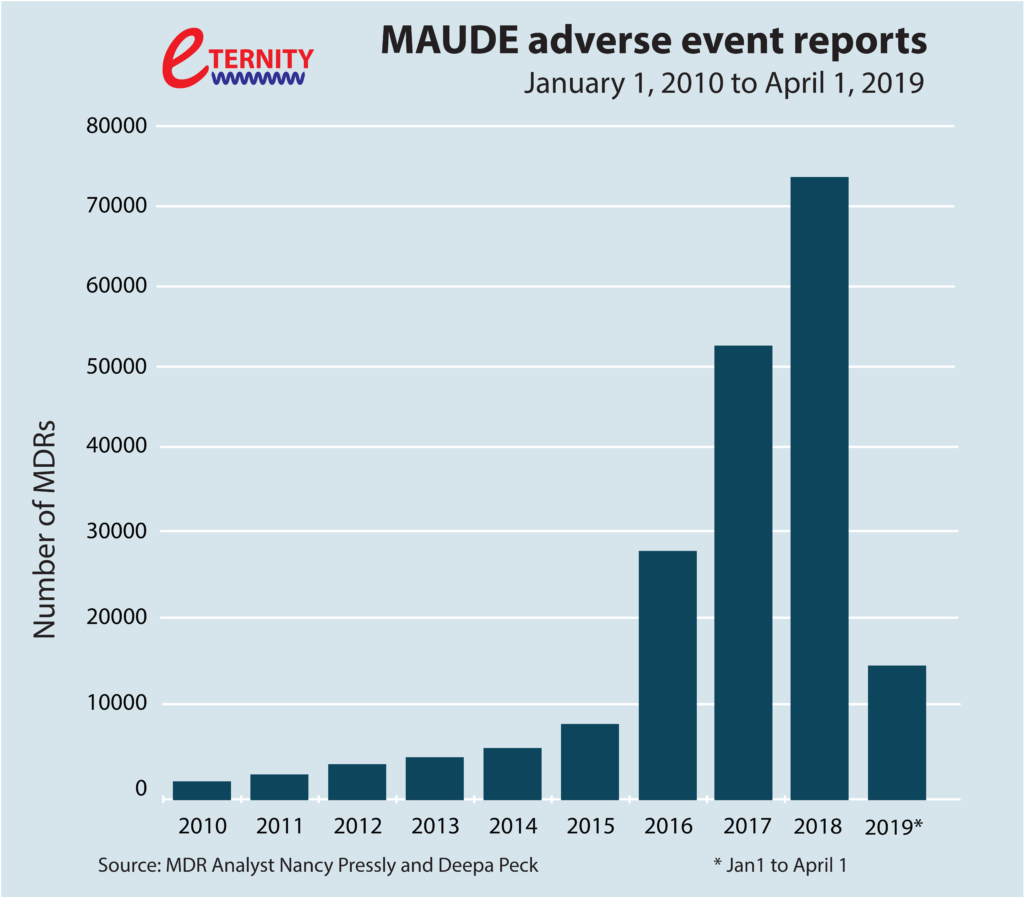
Electromagnetic interference (EMI), familiar to most people as the buzzing sound from a loudspeaker when a mobile-phone call is received, is becoming much more than just an annoyance. This is especially true for the medical-technology industry, one of Europe’s most diverse and innovative high-tech sectors. EMI can obstruct communications, and with equipment such as life-support systems, people’s lives are put at risk. This dangerous situation is made worse by the fact that as companies produce new medical devices that are more effective, provide better care and are more widely available to patients, the problems associated with EMI are multiplying. The numbers of hospital incidents – and even deaths – is increasing (see Figure 1, based on voluntary reports). It is very clear that we need to find new ways to tackle this problem of managing EMI in complex, health-related scenarios.
A “rule-based” approach is no longer sufficient. The conventional approach to dealing with EMI is to use a rule-based approach. This means that we take the existing standards, we read what the rules tell us to do and then we follow the rules. So, in a rule-based approach for a medical device, we would identify early in the design phase the applicable EMI standards, review their technical requirements, resulting in the default application of a set of mitigation techniques (filtering, shielding, cable routing, etc.), and finally conduct testing to demonstrate compliance with those requirements.
Why the rule-based approach is lacking. The rule-based approach instinctively feels right, but it has several serious shortcomings. First, standards do not change very quickly; it takes about 5 years to develop a new standard. Hence, as most of us know from experience, standards always lag behind the technological developments, and so when new devices become available, the rule-based approach just cannot keep pace. Relying only on the rule-based approach means we are not taking into account innovations or new ways of doing things. Second, we have no certainties when it comes to knowing whether the existing standards are really sufficient, especially for a complex and invisible phenomenon like EMI. In contrast, as highlighted in Fig. 1, there have been many situations in which a medical device has met the existing EMI standards, but has failed to operate safely in line with its intended use. Third, a rule-based approach to EMI can actually hinder innovation. In a rule-based approach, manufacturers must seek ways to ensure that their new products comply with existing standards, even when those standards fall short of addressing the specific challenges mandated by changing healthcare demands. Instead of spending precious resources on the development of breakthrough design approaches, time is spent developing less innovative but more compliant designs.
What we need is a “risk-based approach”, which will offer us much higher levels of safety. Medical systems become more complex and we become increasingly dependent on their reliability. A risk-based approach is one where we focus our resources and energy on the inherent risks of the functionalities that our medical systems have and on a constant and continuous drive to reduce those risks to the lowest possible level. A risk-based approach for EMI begins with the identification of the EMI-related hazards that could be potentially related to the device’s use. This task includes listing the subsystems used to produce the medical system, the operational environments and the different scenarios in which patients or operators can be harmed. Next, dedicated measures are taken to eliminate or mitigate these possible hazards. Finally, all the argumentation and evidence are gathered in clear documentation to show to all the stakeholders that the risks have indeed been reduced to the lowest possible level.
The challenges that the actual implementation of such a risk-based approach bring should not be underestimated. Up to now each device had been addressed individually with the goal of ensuring its own protection against EMI, using arbitrary sets of standardized values as a reference. A fundamental shift in design philosophy is to now think in term of scenarios to ensure that a device works safely in its intended environment of use over its lifetime. Each possible interaction with other devices in every type of setting has to be considered. The change is more profound than simply re-enforcing further existing EMI protections. It is about understanding new environments of use, adapting and inventing solutions of protection with respect to new EMI issues, while maintaining the key design characteristics of the medical device. It is also about ensuring a long-term resilience and reliability to constantly changing, increasingly complex EMI scenarios.
An interdisciplinary, risk-based approach. The safer use of medical equipment based on assessing EMI risks requires bringing together expertise from 4 key areas – electromagnetic compatibility (EMC), medical engineering, system safety engineering and risk management – for the implementation of the risk-based approach. Indeed, in this new philosophy, each scenario becomes a new system to engineer. For the risk to be managed through the design life cycle, this system thinking process has to result in adapted EMI protection that respects the specifics of the medical product. This required interdisciplinarity expertise is provided by the ETERNITY consortium.
The law demands a risk-based approach. Regulators are also recognizing the need for a risk-based approach. The recent Blue Guide (which is about the implementation of EU product rules) made an EMI risk-based approach (rather than a conventional, rule-based approach) mandatory for any new piece of equipment. Meanwhile, the specific regulations for medical equipment (MDR and IEC 60601-1-2), which also refer to a risk-based approach, will be mandatory either this year, or within the next few years.
The problem is that many companies in the medical-technology industry as well as the users of medical systems (e.g., hospitals) are struggling with this EMI risk-based approach as there is a lack of understanding and no clearly prescribed risk-assessment methodology in place. Small and medium-sized enterprises (SMEs), which are often not able to cope with such a major shift in approach, make up almost 95% of the medical-technology industry.
This new, risk-based methodology will of course require not only a formalization, but trained specialists to address the complexity of the systems and all the individuals and institutions involved.
ETERNITY is built on the two back-bone principles that it must address all the steps of the risk-based approach and be in line with the trends of the medical technology sector, which means all medical environments and all types of devices. This is to ensure the completeness of the programme in term of research, training, industrial-academia network and societal needs.
