What is a Medical device?
When talking about medical devices, one might think exclusively on equipment available around hospitals and clinics and it would not be wrong. However, medical devices are not available only in hospital or other medical environments, they are everywhere and, sometimes, we don’t even realize we are using one!
‘medical device’ means any instrument, apparatus, appliance, software, implant, reagent, material or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more medical purposes”.
Official Journal of the European Union [1]
These include but are not limited to diagnosis, prevention and monitoring of diseases, treatment or alleviation of an injury.
The reality is that medical devices are more and more present in our lives and take the most different shapes for many different purposes.
Contact lenses, crutches, magnetic resonance machines, hip prothesis, defibrillator… even a simple band-aid! These are all examples of medical devices. Check your house: it is very likely that you will find one.
With the advances in technology that we’ve witnessed, medical devices have inevitably become electronic and more and more accessible to the general public as it is the case of blood pressure monitors or thermometers, for example. It is therefore important, that manufacturers make sure the device will be safe to use in a wide range of scenarios by literally anyone.

For that reason, a product with medical intent must undergo a series of tests and certifications before a manufacturer can place it on the market as a medical device. In Europe, the conformity of a medical device is guaranteed by the CE marking (Conformité Européenne). [2]
The CE marking is an European certification that assures that the device complies with all applicable European Union regulations, both standard and product specific. In healthcare, these depend on the medical equipment which is categorized according to its risk of use in four main classes: Class I, Class IIa Class IIb and Class III. The higher the class, the higher the risk.
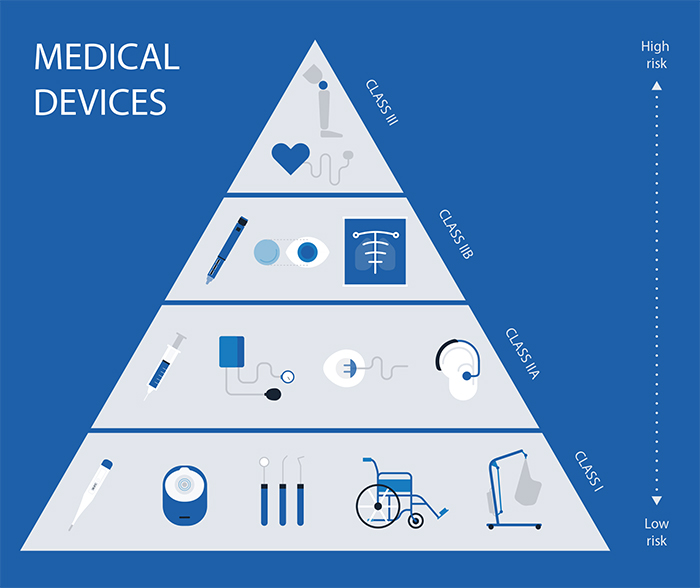
- In Class I we find devices with the least danger of use such as crutches, band-aids and corrective glasses.
- In Class IIa contains devices with a low to medium risk of use, for example, contact lenses and hearing aids.
- In Class IIb we have devices such as contact lenses cleaner, incubators or condoms.
- In Class III we classify the most dangerous devices including catheters, hip implants and heart valves.
Depending on the medical device class, if a failure occurs, the consequences can be more or less dramatic and when it comes to electronic devices, electromagnetic interference must be taken very seriously.

A well-known example of electromagnetic interference is the buzzing sound coming from speakers when telephones nearby are receiving a call. In this case, the consequences are not very bad but imagine being in hospital where its monitoring equipment is giving false information to physicians?
This is the reason why it is so important to tackle this problem: we are testifying a continuously growing number of smart objects with the generalisation of wearables, the Internet of Things and, more recently, 5G technologies which are contributing to an EMI polluted environment even though they were developed to improve our lives.
This means that new electronic equipment in general, and medical devices in particular, must be developed with design techniques that ensure low EMI emissions and also low electromagnetic susceptibility, for it is primordial that new medical equipment can operate safely when sunk in EMI stemming from everyday electronic systems.
After finishing my bachelor’s degree in electrical and electronic engineering I decided I would pursuit my studies in the medical field so that I could have a positive impact in healthcare. Electromagnetic interference is a growing problem that will undoubtedly be a major threat to our medical devices. It is therefore primordial that this issue is properly addressed and right from the very beginning of the development of future medical devices.
In this project, I will focus on establishing new guidelines for the development of medical devices ensuring that design and production methodologies to mitigate EMI are used since during the entire development process. Not only will it guarantee safety of use and compliance with regulations, it will also ease the certification process and improve time to market.


0 thoughts on “Medical devices and Electromagnetic interference”